Product Details
- Product Name: Recombinant Human VEGF (121aa)
- Product Type: Growth Factors & Cytokines
- Catalog Number: Z102115
- Unit Size: 10 µg
- Species: Human (Homo sapiens)
- Gene Symbol: VEGFA
- Gene ID: 7422
- Accession Number: P15693
- Expression System: E. coli
- Molecular Weight: ~14.3 kDa
- Form: Lyophilized powder
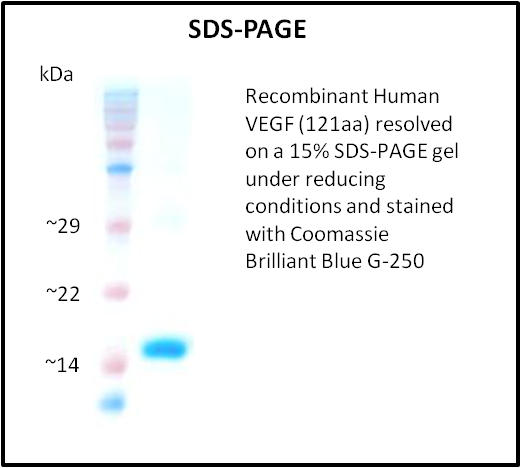
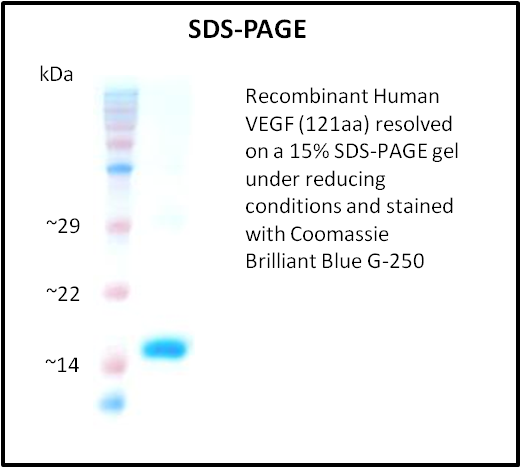
- Purity: > 95% (SDS-PAGE)
- Endotoxin Level: < 1.0 EU/µg (LAL method)
- Appearance: White to off-white lyophilized powder
- Formulation: Lyophilized from 0.2 μm filtered solution in Tris and NaCl, pH 8.0
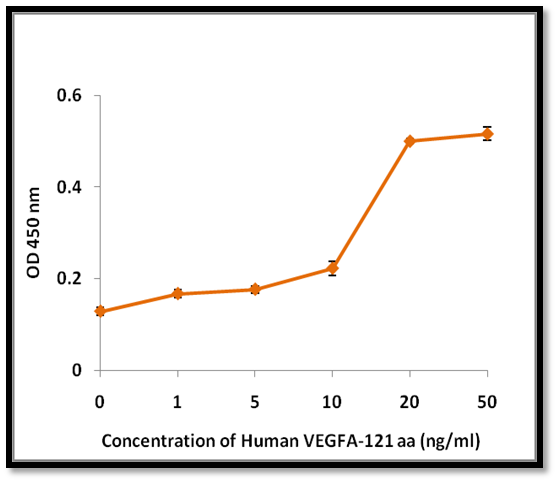
- Function: Induces proliferation of human umbilical vein endothelial cells (HUVEC) with ED₅₀ ~10 ng/mL
- Storage:
- Lyophilized: –70°C (stable for ≥ 12 months)
- After reconstitution:
- 2–8°C (≤ 1 month)
- –20°C (≤ 6 months with carrier protein)
- Avoid repeated freeze/thaw cycles
- Shipping: Dry ice
- Usage: For research use only; not for diagnostic or therapeutic applications
Overview
Recombinant Human VEGF121 is a biologically active, non-heparin-binding isoform of Vascular Endothelial Growth Factor A. This homodimeric glycoprotein plays a central role in angiogenesis and endothelial cell function by stimulating mitogenesis via VEGF receptors. VEGF121, lacking the heparin-binding domain of larger isoforms, is fully soluble and particularly useful for controlled in vitro vascular studies. Expressed in E. coli and highly purified, this recombinant cytokine supports a wide variety of vascular biology, tissue regeneration, and cancer-related applications.
Key Features and Benefits
- Validated Bioactivity: Promotes dose-dependent HUVEC proliferation (ED₅₀ ≈ 10 ng/mL).
- Soluble Isoform: VEGF121 does not bind heparin, offering consistent activity in solution-phase assays.
- High Purity, Low Endotoxin: >95% purity by SDS-PAGE; endotoxin level <1 EU/µg.
- Stable Lyophilized Format: Long shelf-life and flexible storage options.
- Reliable Expression: Recombinant production in E. coli enables scalable research-grade consistency.
Reconstitution Instructions
- Briefly centrifuge vial to collect contents.
- Reconstitute with sterile distilled water to ≥ 0.1 mg/mL.
- Gently swirl to dissolve—do not vortex.
- For downstream use, further dilution in compatible buffer or media is recommended.
- Filter sterilization may be required based on application.
Quality Control (QC)
- Purity validated by SDS-PAGE
- Endotoxin tested via LAL method
- Activity confirmed through HUVEC proliferation assay
Caution
This product is intended strictly for research purposes. It is not suitable for diagnostic, therapeutic, or clinical use in humans or animals.
Disclaimer
- This product is provided for research use only. Not for human or veterinary use. Not intended for clinical, diagnostic, or therapeutic applications.
- The manufacturer assumes no liability for any unauthorized use. It is the user’s responsibility to comply with applicable regulations and safety practices.