Product Details
- Product Name: Recombinant Human CTGF (E. coli)
- Product Type: Growth Factors & Regulatory Proteins
- Catalog Number: Z102025
- Unit Size: 20 µg
- Species: Human (Homo sapiens)
- Gene Symbol: CTGF
- Gene ID: 1490
- Accession Number: P29279
- Source: E. coli
- Molecular Weight: ~11.3 kDa
- Form: Lyophilized powder
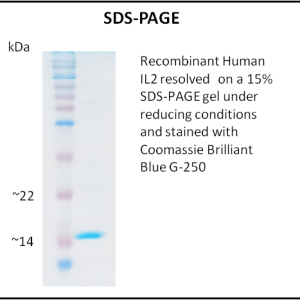
- Purity: >95% (SDS-PAGE)
- Endotoxin Level: <1.0 EU/µg (LAL method)
- Appearance: White to off-white lyophilized powder
- Formulation: Lyophilized from a 0.2 µm-filtered solution containing 0.1% TFA
- Function: Dose-dependent stimulation of proliferation in HUVEC cells; ED₅₀ = 1.0–2.0 µg/mL
- Storage:
- Lyophilized: –70°C (≥ 12 months)
- After reconstitution: 2–8°C (≤ 1 month), –20°C (≤ 6 months with carrier protein)
- Avoid repeated freeze/thaw cycles
- Shipping: Dry ice
- Usage: For research use only; not for diagnostic or therapeutic applications
Overview
Recombinant Human Connective Tissue Growth Factor (CTGF) is a secreted protein involved in regulating cell growth, adhesion, and migration, as well as promoting angiogenesis. Secreted by vascular endothelial cells, CTGF interacts with heparin to stimulate fibroblast and endothelial cell adhesion and migration. The mature human protein (~38 kDa) consists of four functional domains: IGFBP N-terminal, VWFC, TSP type-I, and C-terminal CTCK. This recombinant product corresponds to the CTCK domain, expressed in E. coli in a non-glycosylated form, making it suitable for in vitro studies on angiogenesis, wound healing, and extracellular matrix regulation.
Key Features and Benefits
- Functionally Active: Induces proliferation of HUVEC cells with an ED₅₀ between 1.0–2.0 µg/mL.
- Endotoxin-Safe: Endotoxin level <1.0 EU/µg as determined by the LAL method.
- High Purity: >95% verified by SDS-PAGE for reliable experimental performance.
- Validated Bioactivity: Consistent functional activity demonstrated in endothelial cell-based proliferation assays.
- Stable Format: Lyophilized powder enables extended storage and flexible handling.
Reconstitution Instructions
To ensure optimal activity and protein stability:
- Centrifuge the Vial: Briefly spin to collect the lyophilized powder.
- Add Sterile Solvent: Reconstitute with sterile distilled water to ≥0.1 mg/mL.
- Mix Gently: Swirl gently to dissolve; avoid vigorous agitation or vortexing.
- Dilute for Application: For experiments, further dilute in appropriate buffer with carrier protein.
- Storage After Reconstitution: 2–8°C for ≤1 month or –20°C for ≤6 months. Avoid repeated freeze–thaw cycles.
Quality Control (QC)
- Purity verified by SDS-PAGE.
- Endotoxin level tested by LAL assay.
- Biological activity confirmed via HUVEC proliferation assay.
Caution
This product is intended for research use only. It is not approved for human or veterinary therapeutic or diagnostic applications.
Disclaimer
- For research use only.
- Not for human or veterinary use.
- Not for diagnostic or therapeutic purposes.
- The supplier assumes no liability for the misuse of the product.
- Users are responsible for compliance with all applicable regulations.