Product Details
- Catalog Number: T0056
- Unit Size: 1×10⁶ cells / 1.0 ml
- Species: Human (Homo sapiens)
- Tissue of Origin: Liver
- Donor Information: Adult
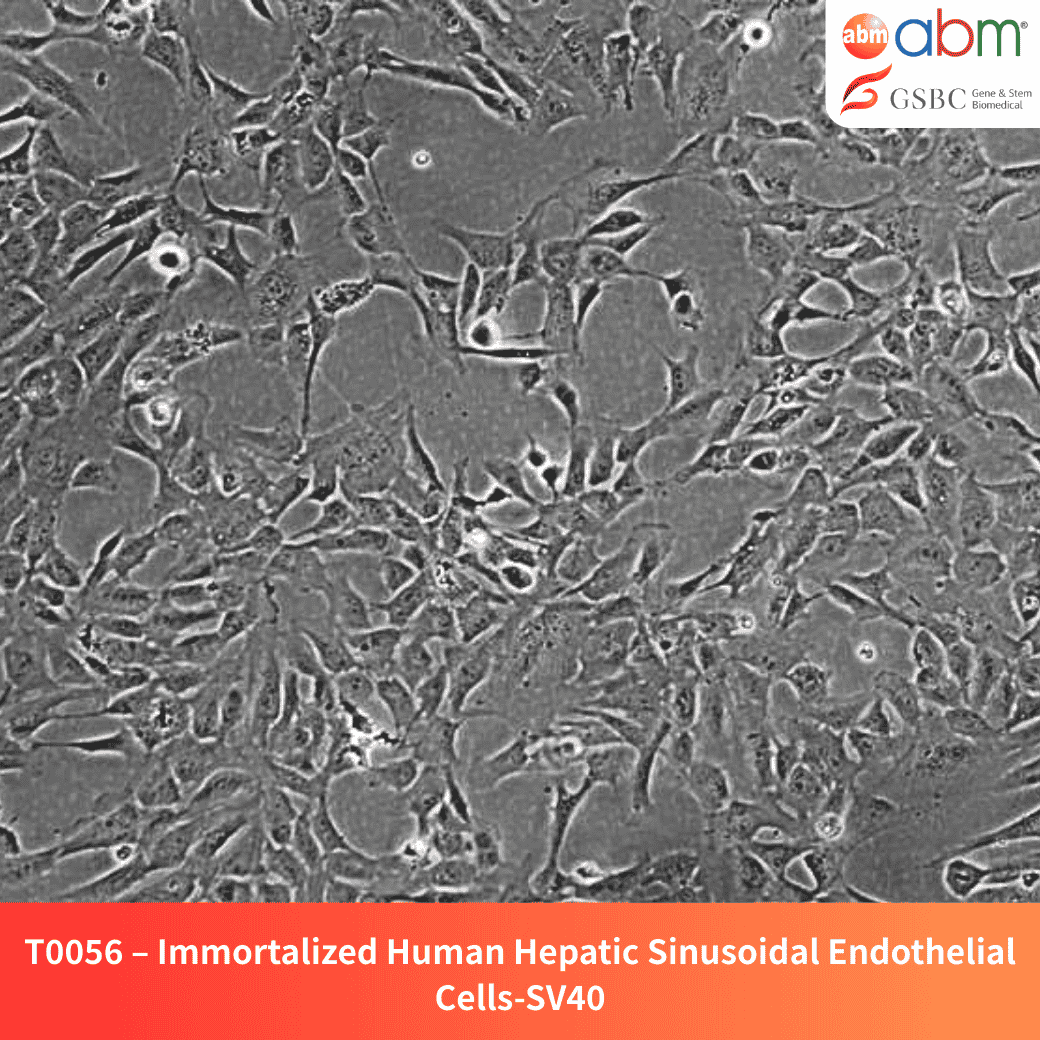
- Cell Type: Immortalized hepatic sinusoidal endothelial cells
- Growth Properties: Adherent, polygonal morphology
- Immortalization Method: Lentiviral transduction with SV40 Large T antigen
- Expression Markers: CK18, CK19, vWF, CD31, VE-cadherin, puromycin resistance
- Biosafety Level: BSL-2
- Storage: Below -130°C (liquid nitrogen vapor phase)
- Shipping: Shipped on dry ice
- Format: Cryopreserved frozen cells
Overview
The Immortalized Human Hepatic Sinusoidal Endothelial Cells (HSEC) provide a robust model for studying liver microvascular function, antigen presentation, and immune tolerance. These cells dynamically regulate porosity in response to zonal stimuli and play a key role in liver homeostasis and regeneration.
Key Features and Benefits
- Liver Immunology & Endothelial Research: Facilitates the study of antigen presentation and immune modulation.
- Dynamic Porosity Regulation: Responds to zonal environmental stimuli, crucial for liver function.
- Drug Discovery & Toxicology: Suitable for testing hepatotoxic compounds and vascular-targeted therapies.
- Consistent & Reproducible: Maintains a stable genetic background for reliable experimental outcomes.
- Adaptability to 3D Culture & Co-Culture: Enhances liver disease modeling and mechanistic studies.
Culture & Handling Guidelines
Recommended Culture Conditions
- Coating: Use Applied Cell Extracellular Matrix (G422). Coat plates at 37°C overnight and wash with sterile PBS prior to use.
- Growth Medium: PriGrow IX (TM019) supplemented with:
- 10% Fetal Bovine Serum (FBS)
- 1% Penicillin/Streptomycin Solution (G255)
- Incubation Conditions: 37°C in a humidified atmosphere with 5% CO₂
- Seeding Density: 20,000 cells/cm²
- Doubling Time: 12–22 hours
Thawing Protocol
- Quickly thaw cells in a 37°C water bath while gently agitating the vial (maximum 2 minutes). Keep the vial cap above the water level to avoid contamination.
- Decontaminate the vial by spraying with 70% ethanol and transfer it to a biological safety cabinet.
- Transfer the cell suspension into a sterile 15mL conical tube containing 5mL of pre-warmed complete growth medium. Centrifuge at 125xg for 5–7 minutes.
- Aspirate the supernatant without disturbing the cell pellet. Resuspend the pellet in fresh complete growth medium and seed into a pre-coated T25 flask.
- Incubate under recommended conditions and allow the cells to recover before passaging.
Subculturing Guidelines
- Aspirate the culture medium and rinse the cells with sterile PBS.
- Add 2–3mL of pre-warmed 0.25% Trypsin-EDTA and incubate at 37°C until cells detach (~2–10 minutes).
- Neutralize Trypsin-EDTA by adding an equal volume of complete growth medium.
- Transfer the cell suspension to a sterile centrifuge tube and centrifuge at 125xg for 5 minutes.
- Aspirate the supernatant and resuspend the cell pellet in fresh complete growth medium.
- Seed at the appropriate density and incubate under recommended conditions.
Cryopreservation Guidelines
- Cryopreservation Medium: Use Cryopreservation Medium (TM024) or complete growth medium supplemented with 10% DMSO.
- Freezing Protocol: Freeze cells at a controlled rate (-1°C per minute) before transferring to liquid nitrogen storage.
Related Products
- Recombinant Human VEGF (121aa) (E. coli) – Z102115
- Recombinant Human TGF Beta-1 (TGFB1) – Z101555
- Recombinant Human PDGFB (E. coli) – Z100355
- Recombinant Human IL6 (E. coli) – Z100555
Disclaimer
- This product is intended for research use only and is not approved for diagnostic, therapeutic, or clinical applications.
- Users are responsible for determining the suitability of this product for their specific application.
- Cells must be handled under Biosafety Level 2 (BSL-2) containment following institutional guidelines.
- No warranties are provided regarding performance beyond the described specifications.
References
- Jung, Hong-Ryul, et al. “Cell spheroids with enhanced aggressiveness to mimic human liver cancer in vitro and in vivo.” Scientific Reports 7.1 (2017): 10499.
- Lee, Ho-Joon, et al. “Elasticity-based development of functionally enhanced multicellular 3D liver encapsulated in hybrid hydrogel.” Acta Biomaterialia 64 (2017): 67-79.
- Buniatian, Gayane Hrachia, et al. “Antifibrotic effects of amyloid-beta and its loss in cirrhotic liver.” Cells 9.2 (2020): 452.
- Lee, Ho‐Joon, et al. “Optimization of 3D hydrogel microenvironment for enhanced hepatic functionality of primary human hepatocytes.” Biotechnology and Bioengineering 117.6 (2020): 1864-1876.