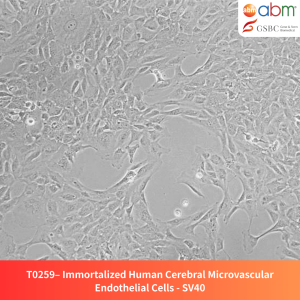
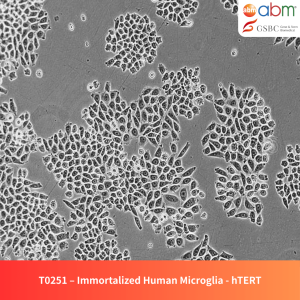
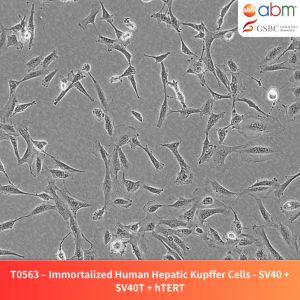
Product Details
- Catalog Number: T0457
- Unit Size: 1 x 10⁶ cells / 1.0 ml
- Species: Human (Homo sapiens)
- Tissue Origin: Heart (Ventricular Cardiomyocytes)
- Donor Information: Female, Ventricle
- Cell Type: Immortalized Cardiomyocytes
- Growth Properties: Adherent, multipolar morphology
- Immortalization Method: Lentiviral transduction carrying SV40T
- Key Markers: GATA-4, ACTN2, ACTN3, MYH2, Puromycin-resistance
- Biosafety Level: BSL-2
- Storage Conditions: Vapor phase of liquid nitrogen (< -130°C)
- Shipping Conditions: Shipped on dry ice
- Format: Cryopreserved frozen cells
- Intended Use: Research use only. Not for human or therapeutic use.
Overview
Immortalized Human Cardiomyocytes – SV40T provide a robust cellular model to investigate cardiomyocyte biology. These cells have been immortalized to sustain extended proliferation while maintaining cardiac-specific markers and functions. They are ideal for applications in cardiovascular research, including signal transduction studies, drug testing, and cardiac toxicity assessment.
Key Features and Benefits
- Extended Culture Viability: Immortalized for prolonged culture without senescence.
- Reproducibility: Ensures consistency in experiments and assays.
- Physiologically Relevant Model: Expresses key cardiomyocyte-specific markers.
- Optimized Growth Conditions: Adapted to specific media and extracellular matrices for enhanced cell viability.
- Adherent Growth Properties: Enables easy handling and maintenance in standard culture settings.
Culture & Handling Guidelines
Recommended Culture Conditions
- Coating: Use PriCoat™ T25 Flasks (G299) or Applied Cell Extracellular Matrix (G422) for optimal adhesion..
- Growth Medium: PriGrow I (TM001) supplemented with:
- 10% Fetal Bovine Serum (FBS)
- 1% Penicillin/Streptomycin (G255)
- Incubation Conditions: Maintain at 37°C in a humidified atmosphere with 5% CO₂.
- Seeding Density: 20,000 – 30,000 cells/cm²
- Doubling Time: ~25–35 hours
Thawing Protocol
- Thaw cells quickly in a 37°C water bath (maximum 2 minutes) while keeping the vial cap above the water level to minimize contamination.
- Decontaminate the vial by spraying with 70% ethanol and transfer it to a biosafety cabinet.
- Add the thawed cell suspension to a sterile 15 mL conical tube containing 5 mL of pre-warmed complete growth medium.
- Centrifuge at 125xg for 5-7 minutes.
- Aspirate the supernatant, gently resuspend the pellet in fresh complete growth medium, and seed into a pre-coated T25 flask.
- Incubate under the recommended conditions until cells recover.
Subculturing Guidelines
- Aspirate the old medium and rinse cells with PBS.
- Add 2-3 mL of pre-warmed 0.25% Trypsin-EDTA and incubate at 37°C until cells detach (~2-10 minutes).
- Neutralize Trypsin-EDTA by adding an equal volume of complete medium.
- Centrifuge at 125xg for 5 minutes, aspirate the supernatant, and resuspend the pellet in fresh growth medium.
- Seed cells at the appropriate density into new culture vessels.
- Incubate under the recommended conditions and monitor for confluency.
Cryopreservation Guidelines
- Cryopreservation Medium: Complete growth medium supplemented with 10% DMSO.
- Freezing Protocol: Freeze at a controlled rate of -1°C per minute, then transfer to liquid nitrogen storage.
Related Products
- Recombinant Human VEGF (121aa) (E. coli) – Z102115
- Recombinant Human Beta-NGF – Z101545
- Recombinant Human TGF Beta-1 (TGFB1) – Z101555
- Recombinant Human IGF1 (E. coli) – Z100385
Disclaimer
- This product is for research use only and is not intended for therapeutic, diagnostic, or clinical applications.
- Performance may vary depending on the user’s culture conditions and adherence to recommended protocols.
- The product must be stored and handled according to the provided conditions for optimal functionality.
- The end-user assumes full responsibility for the suitability of this product for their specific research applications.
- All sales are final. Live cell replacement may be available under specific conditions upon request.
Reference
- Durham, Kristina K., et al. “HDL protects against doxorubicin-induced cardiotoxicity in a scavenger receptor class B type 1-, PI3K-, and Akt-dependent manner.” American Journal of Physiology-Heart and Circulatory Physiology 314.1 (2018): H31-H44.
- Kono, Ken, et al. “Development of selective cytotoxic viral vectors for concentration of undifferentiated cells in cardiomyocytes derived from human induced pluripotent stem cells.” Scientific reports 9.1 (2019): 3630.