Product Details
- Catalog Number: T0280
- Unit Size: 1 x 10⁶ cells / 1.0 ml
- Species: Human (Homo sapiens)
- Tissue: Brain (Fetal, 18 weeks gestation)
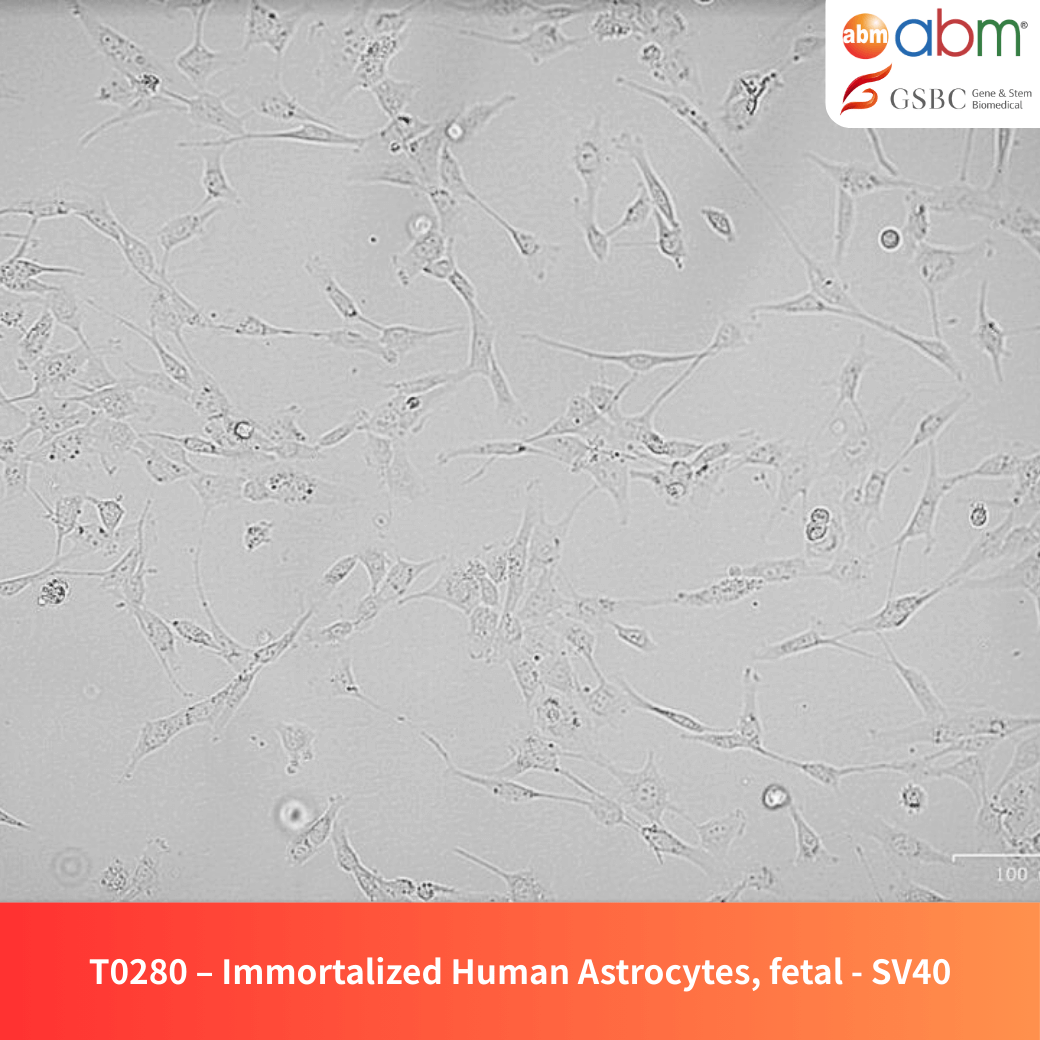
- Cell Type: Immortalized Astrocytes
- Growth Properties: Adherent, multipolar morphology
- Immortalization Method: Genetically modified using SV40 Large T antigen for extended proliferation.
- Expression Markers: NF-M, GDNF and NGF
- Biosafety Level: BSL-2
- Storage: Vapor phase of liquid nitrogen or below -130°C
- Shipping: Shipped on dry ice
- Format: Cryopreserved frozen cells
Overview
Immortalized Human Astrocytes (Fetal, SV40) serve as a stable and reproducible cellular model for investigating astrocyte physiology and function. These cells have been genetically immortalized with the SV40 Large T antigen, ensuring long-term proliferation while maintaining key astrocytic properties. They are ideal for research in neurodevelopment, neuroinflammation, and neurodegenerative diseases.
Key Features and Benefits
- Long-Term Viability: Genetically immortalized to enable extended culture durations.
- Consistent Performance: Provides reproducible results for multiple experimental applications.
- Neuroscience Applications: Ideal for studying astrocyte-neuron interactions, brain injury response, and neuroinflammatory processes.
- Optimized Culture Conditions: Designed to thrive in specialized media and extracellular matrices.
- Easy Handling: Adherent growth properties simplify maintenance in culture.
Culture & Handling Guidelines
Recommended Culture Conditions
- Coating: PriCoat™ ECM T25 Flasks (G999) or Applied Cell Extracellular Matrix (G422) are required for optimal cell adhesion.
- Growth Medium: PriGrow IV (TM004) supplemented with:
- 10% Fetal Bovine Serum (FBS)
- 2 mM L-glutamine (G275)
- 10 ng/mL recombinant human EGF (rhEGF, Z100139)
- 1% Penicillin/Streptomycin Solution (G255)
- Incubation Conditions: 37°C, 5% CO₂ humidified atmosphere.
- Seeding Density: 40,000 – 50,000 cells/cm²
- Doubling Time: 49 – 59 hours
Thawing Protocol
- Thaw cells rapidly in a 37°C water bath for no more than 2 minutes, keeping the vial cap above the water level to prevent contamination.
- Decontaminate the vial exterior by spraying with 70% ethanol before transferring it into a biological safety cabinet.
- Transfer the cell suspension into a 15 mL sterile conical tube containing 5 mL of pre-warmed complete growth medium.
- Centrifuge at 125xg for 5-7 minutes.
- Remove the supernatant without disturbing the cell pellet. Resuspend the pellet in fresh complete growth medium and seed into a pre-coated T25 flask.
- Incubate under the recommended conditions and monitor cell recovery before passaging.
Subculturing Guidelines
- Aspirate the spent medium and rinse cells with PBS.
- Add 2-3 mL of pre-warmed 0.25% Trypsin-EDTA to the flask and incubate at 37°C until cells detach (2-10 minutes).
- Neutralize Trypsin-EDTA by adding an equal volume of complete growth medium.
- Transfer the suspension into a sterile centrifuge tube and centrifuge at 125xg for 5 minutes.
- Remove the supernatant and gently resuspend the pellet in fresh complete growth medium.
- Seed cells at the appropriate density into new culture vessels and incubate under recommended conditions.
Cryopreservation Guidelines
- Cryopreservation Medium: Complete growth medium supplemented with 10% DMSO or Cryopreservation Medium (TM024).
- Freezing Protocol: Freeze cells at a controlled rate of -1°C per minute before transferring them to liquid nitrogen storage.
STR Profiling (Short Tandem Repeat Analysis)
- D5S818: 11,13
- D13S317: 12,12
- TH01: 6,9.3
- AMEL: X,X
Related Products
- Recombinant Human TGFA – Z101935
- Recombinant Human FGF2 (E. coli) – Z101455
- Recombinant Human EGF – Z100139
Disclaimer
- This product is intended for research use only. Not for human or animal therapeutic, diagnostic, or consumption purposes.
- Growth parameters may vary based on user-specific culture conditions. It is recommended to validate in-house conditions before conducting critical experiments.
- All sales are final. No warranty is provided for cell performance outside specified culture conditions.
References
- Choi, Yong-Joon, et al. “PIN1 transcript variant 2 acts as a long non-coding RNA that controls the HIF-1-driven hypoxic response.” Scientific Reports 9.1 (2019): 10599.
- Dozio, Vito, and Jean-Charles Sanchez. “Profiling the proteomic inflammatory state of human astrocytes using DIA mass spectrometry.” Journal of neuroinflammation 15 (2018): 1-14.