Product Details
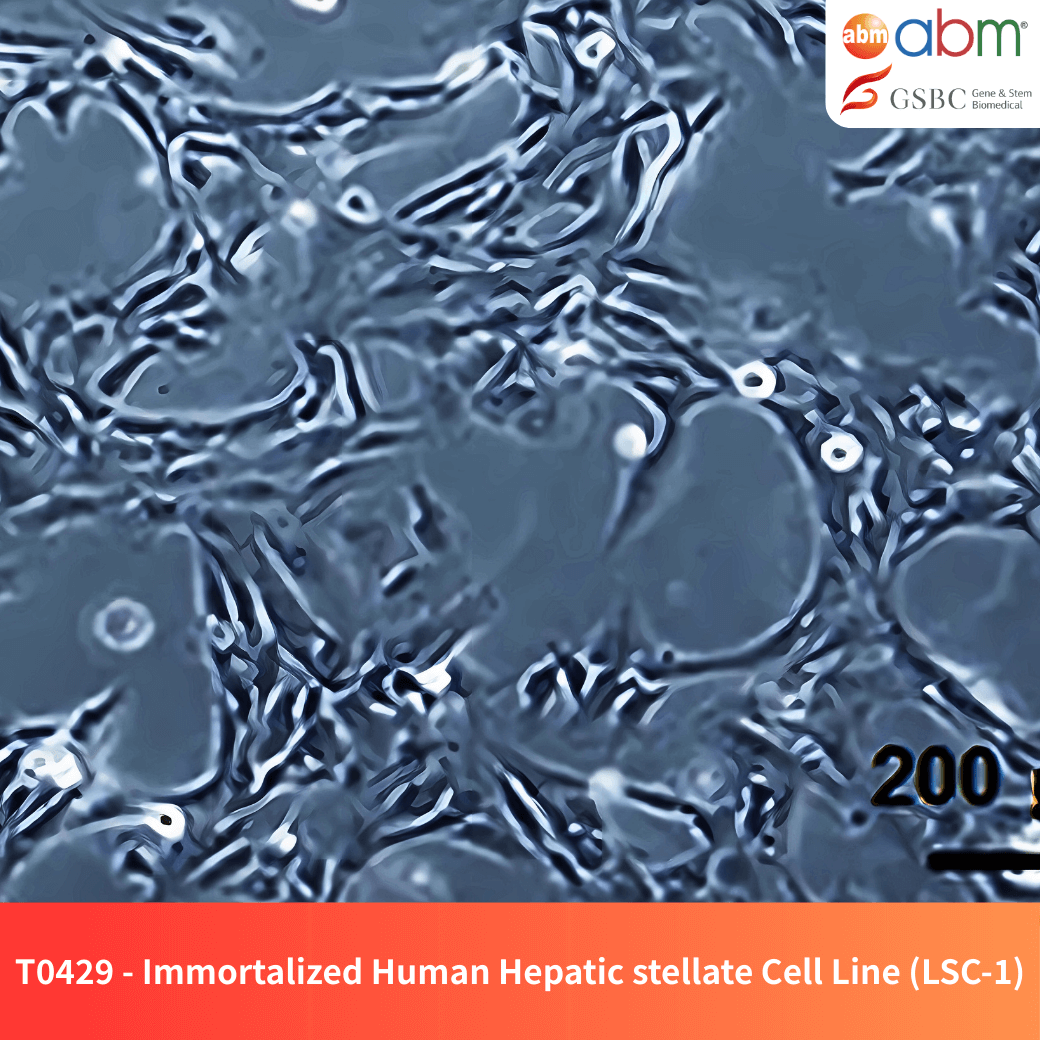
- Catalog Number: T0429
- Unit Size: 1×10^6 cells / 1.0 ml
- Species: Human (Homo sapiens)
- Tissue of Origin: Liver
- Donor Information: Female, normal liver tissue from liver tumor patients
- Cell Type: Immortalized hepatic stellate cells
- Growth Properties: Adherent
- Immortalization Method: Lentiviral transduction with Lenti-hTERT-Neo Virus and Lenti-SV40 lentivirus
- Expression Markers: PDGFRB, GFAP, Desmin, Nestin, α-SMA, Type I Collagen
- Biosafety Level: BSL-2
- Storage: Below -130°C (liquid nitrogen vapor phase)
- Shipping: Shipped on dry ice
- Format: Cryopreserved frozen cells
Overview
The Immortalized Human Hepatic Stellate Cell Line (LSC-1) is a well-characterized cell model engineered for sustained proliferation while preserving key hepatic stellate cell characteristics. It is widely used in liver disease research, fibrosis modeling, and antifibrotic drug screening. LSC-1 cells demonstrate fibrogenic gene expression and can transition between activated and quiescent states under different culture conditions, making them highly valuable for dynamic liver studies.
Key Features and Benefits
- Fibrosis Research: Facilitates the study of fibrogenic pathways and extracellular matrix remodeling.
- Drug Discovery & Toxicology: Suitable for testing antifibrotic compounds and hepatotoxicity assessments.
- 3D Culture & Co-culture Capabilities: Can revert to a quiescent-like phenotype under specialized conditions.
- Reproducibility: Ensures consistency across experiments with a stable genetic background.
- Optimized Growth Conditions: Adapted to specific media formulations for enhanced viability.
Culture & Handling Guidelines
Recommended Culture Conditions
- Coating: PriCoat™ T25 Flasks (G299) or Applied Cell Extracellular Matrix (G422) for optimal cell attachment.
- Growth Medium: PriGrow IX Medium (TM019) supplemented with:
- 10% Fetal Bovine Serum (FBS)
- 1% Penicillin/Streptomycin Solution (G255)
- Incubation Conditions: 37°C in a humidified atmosphere with 5% CO₂.
- Seeding Density: 10,000 – 15,000 cells/cm².
- Doubling Time: Approximately 48 hours.
Thawing Protocol
- Quickly thaw the vial in a 37°C water bath while gently agitating (maximum 2 minutes). Ensure the vial cap stays above the water level to prevent contamination.
- Decontaminate the vial by spraying and wiping with 70% ethanol. Perform all subsequent operations in a biological safety cabinet under aseptic conditions.
- Transfer the cell suspension to a 15 mL sterile conical tube containing 5 mL of pre-warmed complete growth medium. Centrifuge at 125xg for 5-7 minutes.
- Aspirate the supernatant and gently re-suspend the cell pellet in fresh pre-warmed complete growth medium.
- Dispense the cell suspension into a T25 culture flask and incubate at the recommended conditions.
Subculture Protocol
- Aspirate the old medium and rinse cells with PBS.
- Add 2-3 mL of pre-warmed 0.05% Trypsin-EDTA and incubate at 37°C for 2-10 minutes until cells detach. Observe under a microscope to confirm detachment.
- Neutralize Trypsin-EDTA with an equal volume of complete growth medium.
- Transfer the cell suspension to a sterile centrifuge tube and centrifuge at 125xg for 5 minutes.
- Aspirate the supernatant and re-suspend the cell pellet in fresh complete growth medium.
- Seed cells at the desired density into new culture vessels and incubate under the recommended conditions.
Cryopreservation
- Cryopreservation Medium: TM024 or complete growth medium with 10% DMSO.
- Freezing Protocol: Freeze at a controlled rate of -1°C per minute before transferring to liquid nitrogen storage.
Related Products
- Recombinant Human TGF-β1 – Z101555
- Recombinant Human Connective Tissue Growth Factor (CTGF) – Z102025)
- Recombinant Human FGF2 – Z101455)
Disclaimer
- This product is intended for research use only and is not approved for diagnostic, therapeutic, or clinical applications.
- Users are responsible for determining the suitability of this product for their specific application.
- Cells must be handled under Biosafety Level 2 (BSL-2) containment following institutional guidelines.
- No warranties are provided regarding performance beyond the described specifications.
References
- Lee, Ho‐Joon, et al. “In vitro modeling of liver fibrosis with 3D co‐culture system using a novel human hepatic stellate cell line.” Biotechnology and Bioengineering 120.5 (2023): 1241-1253.